Benefits of Surgical N95 over NIOSH N95
 January 09, 2024
4629
January 09, 2024
4629

Medical Fraternity and doctors globally recommend using Masks and Respirators that are FDA approved along with NIOSH N95. Click on the link below and read the blog about what advantages does a Surgical N95 Respirator offer.
The mandatory usage of face masks began with the onset of the Covid-19 pandemic but has continued owing to more than one reason. Our doctors and medical support staff are categorized as people under utmost risk in this pandemic. They have been working in critical environments where they come in contact with patients who might have been infected or carrying the virus along with them. Also we see different variants of the virus whose effects are far ranging and catastrophic to people who may have been vaccinated as well. Hence doctors & Medical Staff need the best respirators which have been designed for such critical environments and tested to perform. Globally US FDA parameters are considered the benchmark for respirators and mask testing. These offer some distinct advantages over the NIOSH N95 product.
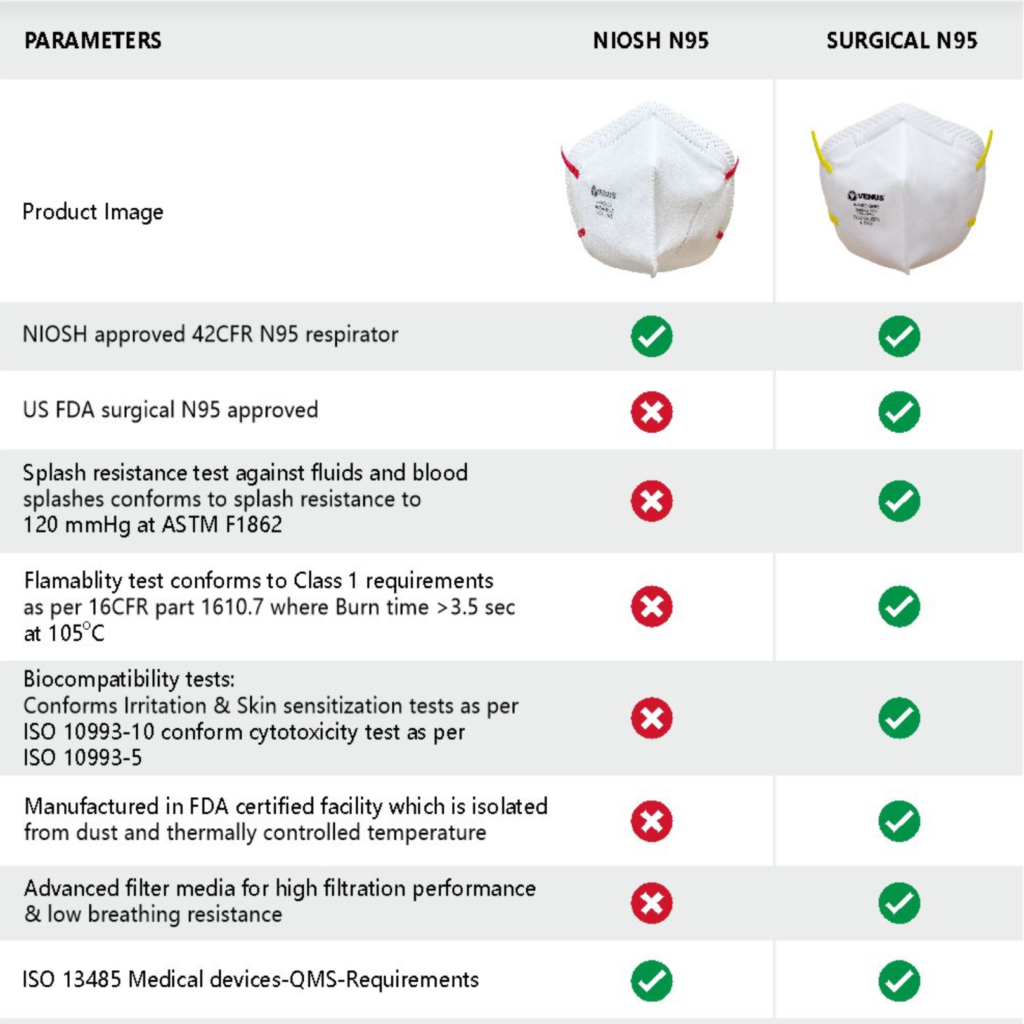
Differences in a NIOSH N95 Respirator and Surgical N95 Respirator –
- NIOSH approved 42 CFR N95 respirator –The testing and certification of several non-powered, air-purifying, particulate-filter respirators have been regulated under a set of regulations under the 42 CFR 84 or part 84 of NIOSH. Both N95 Respirator and the Surgical N95 Respirators pass the tests determined by these regulations.
- Splash resistance test against fluid and blood splashes – The splash resistance test method is performed on Surgical N95 Respirators where a fixed volume of synthetic blood is directed towards the centre of the mask at high velocity. If there is no penetration, the mask passes the test. A Surgical N95 Respirator is splash resistant while the N95 Respirators are not.
- Flammability test – The flammability test is used to determine how easily a medical mask can ignite or burn when exposed to or used near a heat source or fire. The flammability test is passed by the Surgical N95 while a NIOSH N95 Respirator fails the flammability test.
- Cytotoxicity test against skin irritation and allergies –The biological evaluation of face masks conducted through in vitro tests verifies the cytotoxicity, sensitization, and skin irritation when using the mask. The Surgical N95 Respirator shows better results and more comfortable wear.
- Venus V-4400SN95 is one of the first respirators manufactured in India to be approved for Surgical N95. The masks have some unique features over Venus V-4400N95 Respirator which have been used widely by doctors during the pandemic.
- Single packing mask that ensures hygiene and sanitization –When masks are packed as a single unit, it ensures that another mask is not accidentally contaminated while using the first one. It also shows a higher standard of sanitization. The Venus V-4400N95 Respirators are sold in multipacks whereas the V-4400SN95 Respirators are sold in single packs.
- Breathing resistance of 8mm of H2O ensures reduced sweating – A good quality face mask is determined by how low its breathing resistance is, this test is measured by the pressure required to breathe through the fabric and is measured in mm H2The V-4400SN95 Respirators show extremely low breathing resistance of 8mm H2O and hence a reduced amount of sweating inside the mask over the V-4400N95.
- Manufactured in an FDA-certified facility that is isolated from dust and thermally controlled temperature – Both the V-4400N95 and the V-4400SN95 Respirators are manufactured in facilities that are FDA certified, the facilities are isolated from dust and pollution as the manufacturing floor is centrally air conditioned.
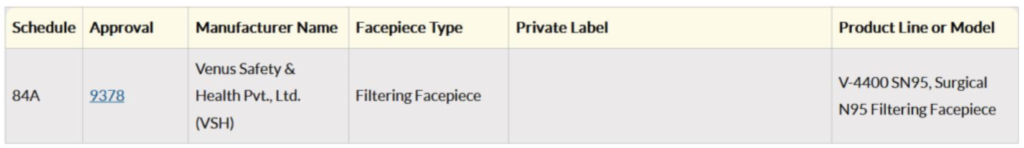
Thus, we have seen some distinct parameters over which a Surgical N95 Respirator is more suitable for doctors over an N95 Respirator. Each masks have the Surgical N95 approval number and its validity can be checked on the US Government CDC website Link https://wwwn.cdc.gov/NIOSH-CEL/. Venus V-4400SN95 which is a Surgical N95 has a unique approval number for which the results displayed on the US Government CDC Website will be as follows
Venus V-4400SN95 is available to doctors through our network of channel partners across India. Get in touch with Venus team today to find out more.



